Molecular level reaction engineering to improve selectivity and reduce deactivation in the methanol-to-olefins process
Molecular level reaction engineering to improve selectivity and reduce deactivation in the methanol-to-olefins process
Promotor(en): V. Van Speybroeck /25308 / Nanoporous materialsBackground and problem
Ethene and propene are among the most important platform chemicals in industry and are typically produced via steam cracking. However, the strive to use more sustainable non-petroleum feedstocks has incentivized the move towards alternative processes. In this context, methanol-to-olefins (MTO) conversion is a promising technology that, at present, is being rolled out on industrial scale. Methanol is contacted with an acid zeolite catalyst that triggers the production of olefins and heavier hydrocarbons. A major issue for the widespread use of this technology is catalyst stability. Fast formation of carbon deposits during operation leads to frequent oxidative regeneration treatments which significantly limits process efficiency. Ideally, molecularly engineered catalysts can be developed which are both resistant towards coke formation but also enable to steer the light olefin selectivity in the desired direction. Various strategies have been developed to achieve this goal, such as co-feeding water, optimizing the process parameters or adjusting material properties like crystallite size and acid site density. [1-2] While some approaches were successful, fundamental understanding of the governing mechanisms leading to aromatics or deactivating species remains limited. Nevertheless, such insight is necessary to develop next-generation catalysts for the methanol-to-olefins conversion.
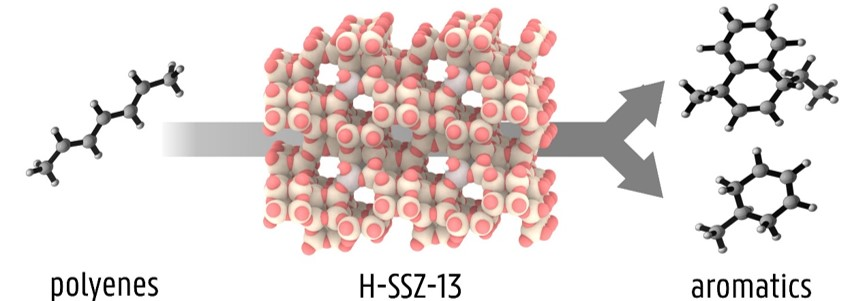
Recently, we unraveled that polyenes are formed at the onset of deactivation in the MTO process on zeolite H-SSZ-13. [3] Using a combination of Raman spectroscopy and molecular simulations we discovered that such polyene species are among the direct culprits for (poly)aromatics formation, as they can undergo fast cyclization (Figure 1). An important factor that has not been considered so far, is the influence of zeolite acidity on the cyclization reactions. The acidity of a zeolite can be moderated by introducing extra-framework species in the pores, such as calcium (hydr)oxide clusters. [2] The change in acid strength is expected to influence the rate of the cyclization reactions, possibly slowing down the process and improving the catalyst lifetime. However, the extent of this effect is not yet clarified and requires a further joint theoretical/experimental investigation.
Goal
During this master thesis you will apply computational methodologies to investigate how the main mechanistic pathways to from polyenes and (poly)aromatic species in the MTO process are affected by acid site modulation through the introduction of extra-framework metallic clusters. Considering that these species also deplete the zeolite cavities of free space for the reacting molecules in an extent that depends on the framework architecture, the study will be performed considering the two archetypal zeolite employed in the MTO process, namely H-ZSM-5 and H-SSZ-13. You will start by assessing the role of Ca2+ doping on the stability of important reaction intermediates and on the energetics of selected elementary routes for aromatics formation, like polyene cyclization through electrocyclic rearrangement or Diels-Alder type reactions. Afterwards, other metal (oxide) modifications of the zeolite acid sites will also be considered. This way, you will evaluate how active site engineering of the catalyst can have a beneficial effect on both the light olefin selectivity and catalyst stability. Considering that for some of the reactions a more accurate description of entropic effects will be necessary, a combination of static ab initio techniques and dynamic simulations will be employed to fully account for the mobility of the adsorbed molecules and the flexibility of the catalyst.
The Center for Molecular Modeling (CMM) has a proven track record in performing advanced molecular simulations. The student will be actively coached by experienced researchers to get acquainted with all techniques that are required to tackle the problem at hand. The computational power required for the simulations will be ensured by CMM access to the main national supercomputing infrastructures. This research topic is of extreme interest in the current catalysis research and will therefore be conducted in close contact with our experimental collaborators at the University College of London. Their exceptional expertise in the in situ spectroscopical characterization of the MTO catalyst will allow to directly link the theoretical insights with experimental observations.
List of figures:
Figure 1: Schematic representation of the transformation of polyenes into aromatics on a H-SSZ-13 zeolite catalyst.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, Zeolites, Methanol-to-olefins, AromaticsReferences
[1] Arora, S. S. et al. Nat. Catal. 2018, 1, 666-672.
[2] Yarulina, I. et al. Nat. Chem. 2018, 10, 804-812.
[3] Lezcano-Gonzalez, I. et al. Nat. Mater. 2020, 19, 1081-1087.
