Micropores or Mesopores? The specific role of surface chemistry in hierarchical zeolite catalysts for methanol-to-hydrocarbon conversion
Micropores or Mesopores? The specific role of surface chemistry in hierarchical zeolite catalysts for methanol-to-hydrocarbon conversion
Promotor(en): V. Van Speybroeck /28130 / Nanoporous materialsBackground and problem
Acid-zeolite catalysts, already ubiquitous in industry, hold promise for future technologies that shift the foundation of the chemical industry from fossil-based feedstocks to renewable sources [1]. Thanks to its versatility, zeolite-catalyzed methanol-to-hydrocarbon (MTH) conversion is an encouraging alternative for the synthesis of light olefins, paraffins, and aromatics that serve as the bedrock of chemical commodity, fuel, and polymer production. The product distribution of MTH processes can be tuned through alterations to the zeolite topology and acidity, in addition to reaction conditions. Furthermore, the crystal morphology and porosity of the zeolites have also demonstrated strong influence on both the product selectivity and catalyst deactivation rates [2]. Evidently, surface chemistry in mesopores and external surfaces may play a significant role in overall catalyst performance. However, the heterogeneous nature of solid-surfaces challenges experimental characterization and little is known about the relative abundance of external surface sites and their distinct reactivity.
Hierarchical pore zeolites, consisting of a combination of micropores and mesopores, display beneficial characteristics for MTH conversion with improved product selectivities and reduced coking rates [3]. In addition to the crystalline microchannels, mainly containing isolated Si-(O)H-Al Brønsted acid sites, these zeolite frameworks also exhibit a considerable amount of external and mesopore surface area, which may contain a diverse range of chemical motifs (Figure 1).
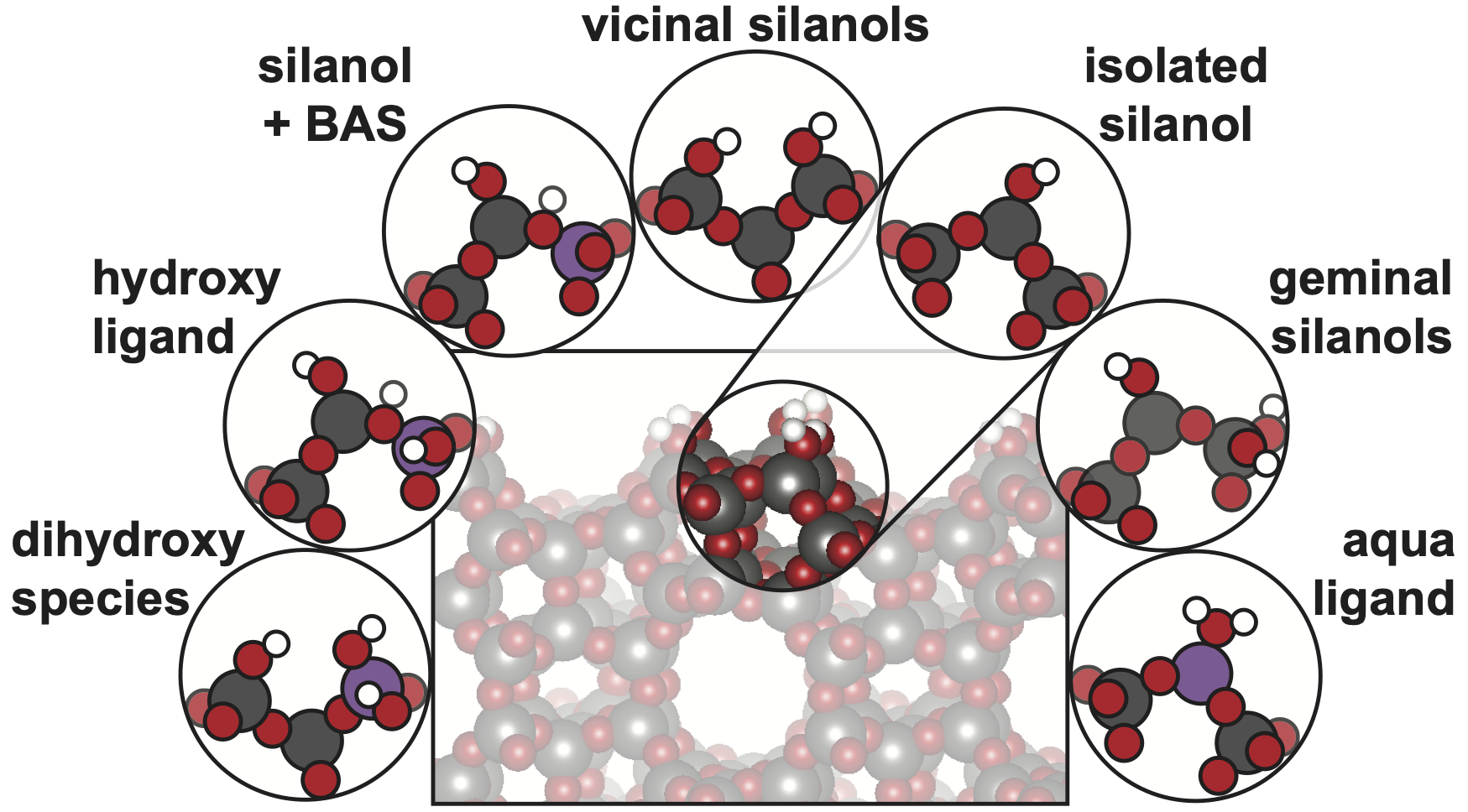
Figure 1: Silanol surface termination of an MFI zeolite catalyst and depictions of a range of possible chemical motifs that may be present at mesopore and crystallite surfaces and within exposed pore micropore mouths.
The presence of these different sites results in altered adsorption modes, different proton affinities, and the observation of pore mouth catalysis [4]. Indeed, the acidity and reactivity of an active site is defined by the local chemical environment, which is markedly different at a terminating surface relative to the inside of the microchannels [5]. The overall catalytic efficacy of a nanoporous materials stems from the combination of surface and internal reactivity. However, the distinct role of various surface facets and functionalities on the overall MTH process is not yet known, and is an essential element to guide future catalyst development.
Goal
This master thesis project aims to understand how the chemistry of terminating surfaces at internal mesopores and crystallite surfaces impacts the selectivity and deactivation of MTH catalysts. You will explore the stability and reactivity of acidic motifs on MFI terminating surfaces employing advanced computational zeolite models to account for the presences of mesoporosity and external surfaces. After a detailed characterization of the active sites and surface chemical motifs, the second phase will consist of assessing the stability and adsorption behavior of common MTH intermediates at the different sites. Finally, you will compute activation barriers and recover experimentally relevant rate constants for key mechanistic steps in the MTH process. To achieve this goal, a combination of static modeling procedures and state-of-the-art molecular dynamics simulations will be applied to properly capture how the interactions of products and reactants with zeolites surfaces are impacted by different surface facets, terminal groups, and Al-siting. The obtained knowledge on the nature and reactivity of various catalytic active sites will provide the necessary insight for identifying design strategies to improve catalyst selectivity and lifetime for the industrial MTH process.
The Center for Molecular Modeling has built up vast expertise in modeling of zeolite catalysis with advanced simulation techniques. The student will be actively coached to get acquainted with the multitude of available techniques to tackle the proposed problem and will be involved in the running collaboration with our leading experimental partners on this topic.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsHeterogeneous Catalysis, Zeolites, Surface chemistry, MTH conversionReferences
[1] Nat. Catal. 2018, 1, 398-411.
[2] Nat. Comm. 2014, 5, 3922.
[3] ACS Catal. 2019, 9, 4, 2880–2892
[4] ChemCatChem 2017, 9, 2176–2185.
[5] ACS Catal. 2020, 10, 5, 3297–3312
