Growing new ice-like structures to capture energy carrier molecules
Growing new ice-like structures to capture energy carrier molecules
Promotor(en): V. Van Speybroeck /28141 / Nanoporous materialsBackground and problem
One of the remaining crucial challenges to enable a green economy is to transport the molecules that carry the energy – methane or hydrogen gas – in an efficient yet safe way. Especially for the hydrogen economy, it is crucial that hydrogen gas, which is highly flammable in a compressed state, is transported safely so to prevent incidents. In this respect, clathrates may form the ideal solution. Clathrates are water crystals – similar to ice – but unlike ice they contain cavities that are large enough to encapsulate guest molecules such as hydrogen gas and methane (see Figure 1). Since these cavities ensure that different energy carrier molecules are spatially separated even under the high energy densities needed to make this process economically feasible, clathrates are currently receiving exceptional interest from researchers and industry alike.1
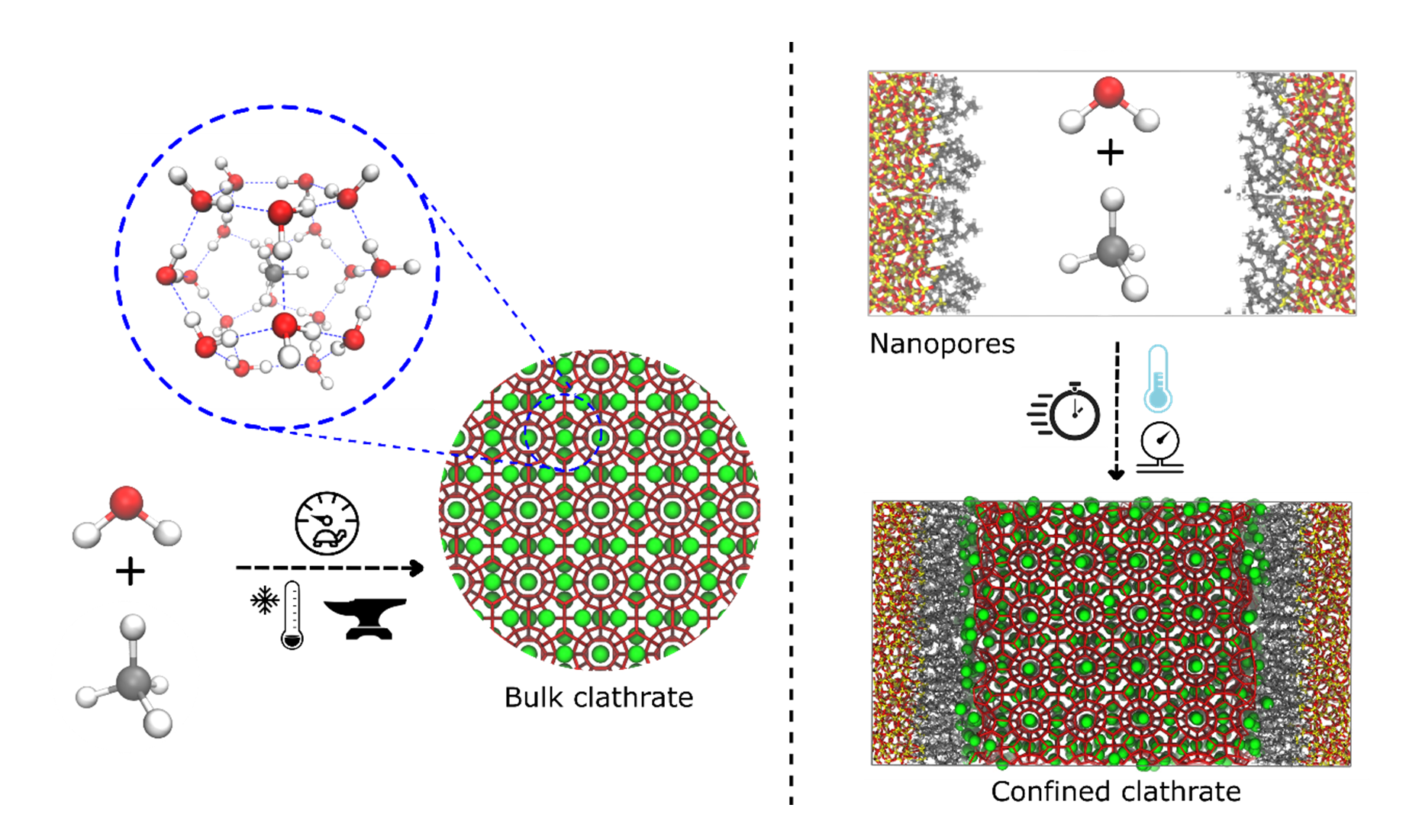
Figure 1: Structure of a methane clathrate, formed when water molecules organize periodically around methane molecules. While bulk clathrates (left) require high pressures and low temperatures to nucleate, these conditions can be eased to obtain a faster nucleation in hydrophobic nanoporous materials (right).
Clathrates, specifically so-called methane clathrates in which water molecules organize themselves around methane molecules, can be formed naturally and are for instance found in abundance on the ocean floor. However, one of the main challenges to produce clathrates on an industrial scale are the harsh conditions required to grow them – requiring both a low temperature and a high pressure – in combination with the slow kinetics of the nucleation process. Hydrophobic nanoporous materials can speed up the formation process of clathrates and ease these conditions, as their hydrophobic internal surface is able to promote the clathrate nucleation and growth (see Figure 1). While some aspects of the nucleation mechanism are already known for bulk clathrates,2 it is unclear how this process is altered when the clathrate forms inside the cavities of nanoporous materials. In bulk, methane molecules are observed to aggregate in certain regions, where molecular collisions allow the methane molecules to partially shed off their hydration layer, which can subsequently trigger the clathrate nucleation.2 In the hydrophobic nanoporous materials, however, the interactions between the water and methane molecules on the one hand and the hydrophobic surface on the other hand will alter the nucleation process, although further insight into this mechanism is still lacking. As a result, it remains unclear which hydrophobic materials are best suited to promote clathrate nucleation or how to improve on these materials.
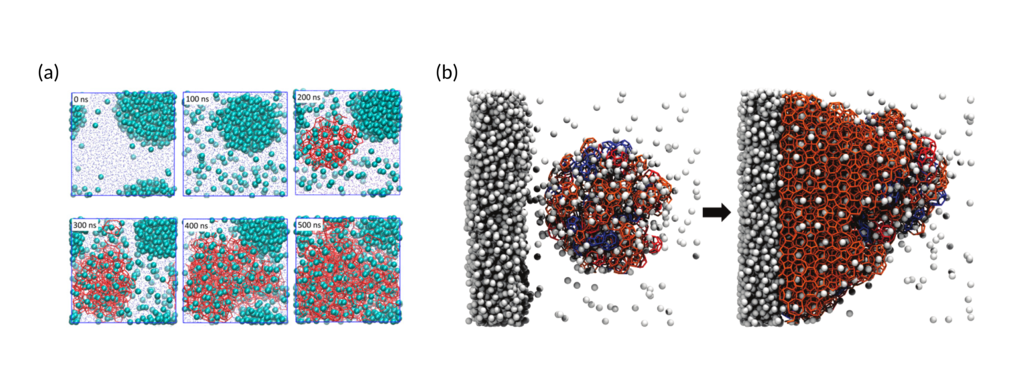
Figure 2: (a) Nucleation process of bulk methane clathrates and (b) the growth of a clathrate from an amorphous nucleus. Figure adapted from refs. 2 and 4 with permission from the National Academy of Sciences of the USA and the American Chemical Society, respectively.
Goal
The goal of this thesis is to unravel the nucleation and growth mechanism of clathrates inside the pores of different hydrophobic materials and to derive structure-property relationships that can be used to establish design principles to promote clathrate nucleation. In a first task, mesoporous hydrophobic materials will have to be constructed and parametrized, in close feedback with our experimental partners who synthesized these materials. Since these materials are amorphous, an appropriate methodology will have to be adopted, akin to polymer growth, to obtain realistic yet dense amorphous materials. Afterwards, appropriate mesopores will be created in these dense silica materials in which the clathrates can then nucleate. These internal cavities will be decorated by different hydrophobic moieties, as the length and degree of hydrophobicity was demonstrated to strongly impact the clathrate structure.3
In a second task, the nucleation of clathrates in the hydrophobic materials constructed in the first task will be investigated via molecular dynamics simulations which follow the nucleation process in real-time. Given that the systems under consideration will contain many thousands of atoms, the use of GPU-accelerated software will be indispensable; these simulations will therefore be performed on our GPU high-performance clusters. For pressures, temperatures, or mixtures for which the clathrate nucleation would still be prohibitively long, enhanced sampling protocols will be examined to speed up the nucleation process. Once the nucleation mechanism is thoroughly analyzed, the optimal conditions for the pressure, temperature, and composition of the mixture can be determined by screening different setups to extract relevant design criteria and thus tailor the hydrophobic materials for efficient gas storage in clathrate hydrates, thereby furthering the design of new hydrogen storage materials.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]Keywordsclathrate nucleation, materials development, gas storage, energy storage, hydrogen gas, structure-property relationshipsReferences
1A. Gupta, G.V. Baron, P. Perreault, S. Lenaerts, R.-G. Ciocarlan, P. Cool, P.G.M. Mileo, S.M.J. Rogge, V. Van Speybroeck, G. Watson, P. Van Der Voort, M. Houlleberghs, E. Breynaert, J.A. Martens, J.F.M. Denayer, Energy Storage Materials 41: 69, 2021.
2L. Lie, J. Zhong, Y. Yan, J. Zhang, J. Xu, J.S. Francisco, X. C. Zeng, Proc. Natl. Acad. Sci. U.S.A. 117: 24701, 2020.
3P.G.M. Mileo, S.M.J. Rogge, M. Houlleberghs, E. Breynaert, J.A. Martens, V. Van Speybroeck, J. Mater. Chem. A 9: 21835, 2021.
4L.C. Jacobson, V. Molinero, J. Am. Chem. Soc. 133: 6458, 2011.
