Chemische kinetiek van Lewiszuur gekatalyseerde reacties in metal-organische roosters
Chemische kinetiek van Lewiszuur gekatalyseerde reacties in metal-organische roosters
Promotor(en): V. Van Speybroeck /MM_13_NANO01 / Nanoporous materialsMetal-Organic Frameworks (MOFs) are - just as zeolites - ordered nanoporous materials with pore sizes between 0.5 and 2 nm. In contrast to zeolites, which have a purely inorganic character, MOFs constitute a new class of porous materials that exhibit a truly hybrid character, since both inorganic and organic moieties are needed to build up their framework. While catalytic conversions and separations are the most frequently used industrial applications of zeolites, future MOF applications lie more in adsorption, storage and separation of gas mixtures (H2, CO2 or CH4), and nowadays their importance in catalysis is steadily increasing. Although catalysis is potentially one of the most important applications of these exciting class of materials, design of suitable MOFs for catalytic applications remains a challenge to be explored.
With the aid of molecular modeling and experimental work, we unraveled that the active metal sites in Zr-terephthalate (UiO-66 type materials) need to be partially decoordinated to be active. In other words, missing terephthalates create these active sites. New materials were made by inserting electron-withdrawing substituents on the terephthalates and tested for the citronellal cyclization (reaction 1, displayed in Figure 2). Both the catalyst activity and selectivity towards the desired isopulegol product increased significantly. The best result was obtained for nitro-substituents and was further studied by molecular modeling on two types of cluster models (cluster representation in Figure 1).2
In this thesis project, we will computationally study other types of reactions such as the Meerwein-Ponndorf-Verley reduction of 4-tert-butylhexanone (reaction 2, Figure 2). For reaction 2 both the carbonyl compound and the alcohol need to be simultaneously activated on the active site. Without modulation with TFA , no conversion is observed experimentally. With TFA, adjacent active sites are formed, creating a more open structure and allowing the reaction (experiments performed at the Center for Surface Science and Catalysis (KULeuven, Prof. D. Devos)). It is challenge for molecular modeling to be able to unravel these experimental findings. Active sites of various substituted UiO-66s will be modeled and the reaction mechanism, activity and selectivity examined (Figure 2).
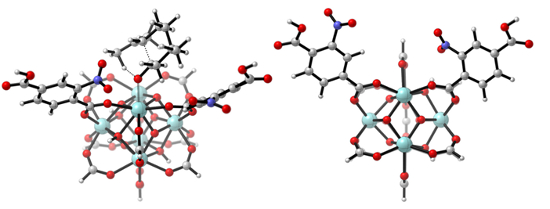
Fig. 1: LEFT: side view of UiO-66-NO2 cluster model for the citronellal cyclization; RIGHT: top view on the modeled active site. 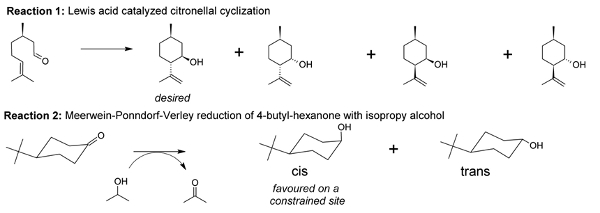
Fig. 2: Overview of reactions that can be studied to unravel the active site topology of the UiO-66-X materials. References:
[1] J. H. Cavka, et al., J.Am.Chem.Soc., 2008, 130, 13850-13851.
[2] Vermoortele, F.; Vandichel, M. et al., Angewandte Chemie-International Edition 2012, 51, 4887.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsMetal-organic frameworks, Chemical kinetics, Nanoporous materials


