Chemische kinetiek van basisch gekatalyseerde reacties in metaal-organische roosters
Chemische kinetiek van basisch gekatalyseerde reacties in metaal-organische roosters
Promotor(en): V. Van Speybroeck /MM_13_NANO02 / Nanoporous materialsMetal-Organic Frameworks (MOFs) are - just as zeolites - ordered nanoporous materials with pore sizes between 0.5 and 2 nm. In contrast to zeolites, which have a purely inorganic character, MOFs constitute a new class of porous materials that exhibit a truly hybrid character, since both inorganic and organic moieties are needed to build up their framework. While catalytic conversions and separations are the most frequently used industrial applications of zeolites, future MOF applications lie more in adsorption, storage and separation of gas mixtures (H2, CO2 or CH4), and nowadays their importance in catalysis is steadily increasing. Although catalysis is potentially one of the most important applications of these exciting class of materials, design of suitable MOFs for catalytic applications remains a challenge to be explored.
This thesis subject is focused on reactions in basic-MOFs, such as MOF-74(M) (Figure 1). MOF-74 is constructed from dihydroxy terephthalate and a cation of valence +2 (M= Mg, Ni, Co, Zn, Mn, Cu and Fe). These MOF-74s contain in fact both unsaturated Lewis acid metal sites as well as Brønsted basic oxygen sites (collaboration with COK, Prof. D. De Vos - KULeuven).
Initial experiments on MOF-74 variants have already shown the modulating effect of the metal on the conversion of the Knoevenagel condensation of malonitrile and benzaldehyde (reaction 1, Figure 2). The activity of the MOF-74 materials did also correlate with proton affinity calculations. However, the exact mechanism of the base catalyzed reactions (Figure 2), still needs to be determined.
Within this thesis attention will be given to MOF-74 variants and their use as base catalysts. Three reactions will be studied that require - as a first step - the deprotonation of one reactant at a base site (Reactions 1, 2, 3), forming a carbanion adsorbed on an adjacent metal site. Reaction 1 should go faster than reaction 2, based on the pKa of the nitriles (pKa(malonitrile)=11, pKa(ethyl cyanoacetate)=9) that deprotonate before reaction. A Michael 1,4-addition (reaction 3, Figure 2) will also be studied as this type of reaction belongs to the larger class of conjugate additions and is one of the most useful strategies for the mild formation of C-C bonds. This reaction can be seen as a nucleophilic addition of a carbanion of ethyl cyanoacetate with methyl vinylketon.
A modeling study will be performed to provide insight into the detailed reaction mechanism and the nature of the active site. Such study will allow to unravel the governing factors to enhance the catalytic activity. A variety of theoretical techniques (encompassing cluster and periodic calculations) are available at the Center for Molecular Modeling (CMM).
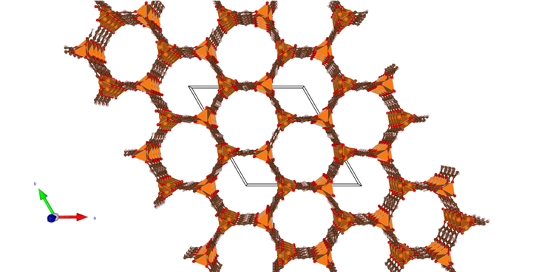
Figure 1: The hexagonal honeycomb structure of MOF-74. 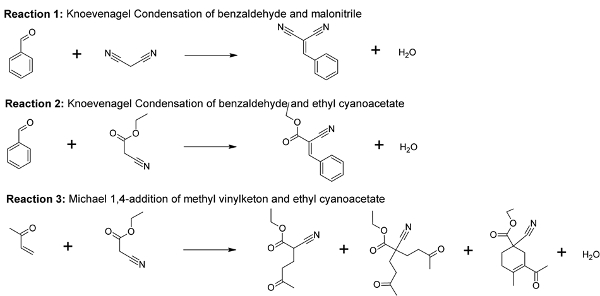
Figure 2: Reactions that can be studied to unravel the active site topology of MOF-74 materials.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsChemical kinetics, Metal-organic frameworks, Base-catalyzed reactions


