Abstract
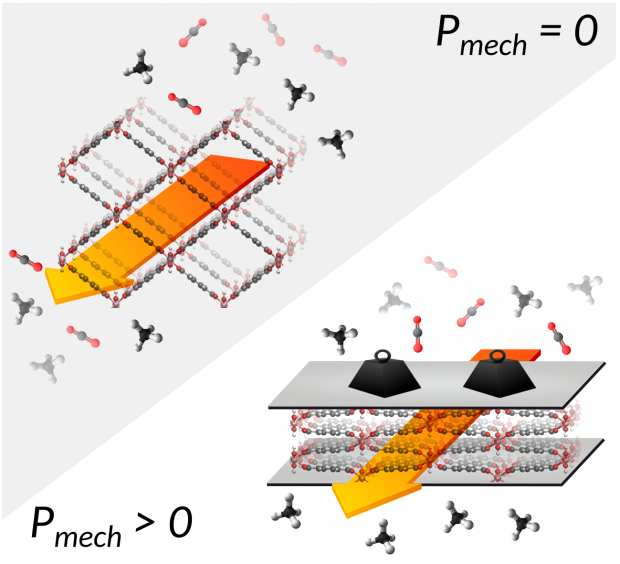
The coadsorption of CO2/CH4 in the breathing MIL-53(Cr) under the application of an additional mechanical pressure is investigated through the use of an extended thermodynamic mean-field model. The focus is on the breathing behavior, negative gas adsorption (NGA), and selective adsorption of CO2 as well as to what degree the application of mechanical pressure influences this behavior. To this end, phase diagrams, coadsorption isotherms are constructed and the CO 2 /CH 4 selectivity is computed in terms of the vapor pressure of methane and carbon dioxide as well as the mechanical pressure. As a result, it was found that NGA can be induced by coadsorption of CO2 /CH4 gas mixtures with certain molar compositions. Finally, a specific adsorption/desorption cycle, which includes the application of an additional mechanical pressure, is proposed to allow for an increased CO2/CH4 selectivity as well as for an expected less energy demanding CO2 desorption step.
