Dosimetric characteristics of different types of saccharides: An EPR and UV spectrometric study
Abstract
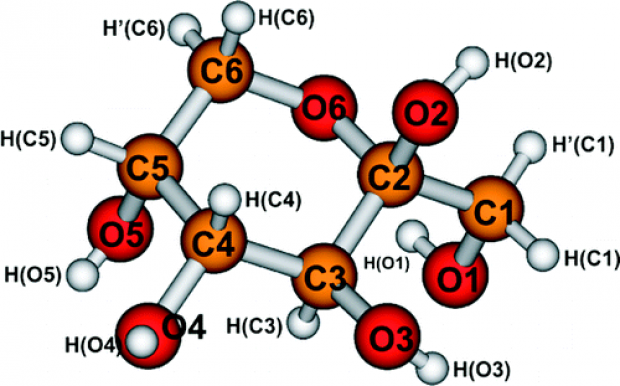
The time stability and dose response of the free radicals produced in various types of “less-studied” mono- and disaccharides by γ-radiation is studied by EPR (Electron Paramagnetic Resonance) and UV spectrometry. The time evolution of the shape of the EPR spectra of irradiated saccharides is investigated from 5 min to 5 months after irradiation. The intensity of the stable EPR signal is studied as a function of the absorbed γ-dose in the range 0.5–20 kGy. Aqueous solutions of irradiated solid saccharides exhibit a UV absorption maximum in the range 250–290 nm. A linear dependency is found between the magnitude of the UV absorption maximum and the absorbed γ-dose. The time stability of the UV absorption maximum is also studied for every saccharide. The results are compared with those obtained for irradiated sucrose.

 Open Access version available at
Open Access version available at