Multi-reference study of the metal-Pi organic linker bond in metal-organic frameworks
Multi-reference study of the metal-Pi organic linker bond in metal-organic frameworks
Promotor(en): V. Van Speybroeck, D. Van Neck /16FUND05 / Many-particle physicsMetal-organic frameworks (MOFs) are crystalline porous materials, which are very promising for applications such as gas storage and drug delivery. Since their discovery about 10 years ago, MOFs have attracted a lot of attention due to their peculiar metal-organic linker bonds. The organic linker is mostly built from a π-conjugated system (Figure 1). One could think that any metal of the periodic system could be combined with any organic linker molecule. However, not all combinations of metals and organic linkers are feasible. To understand the stability of these materials, the metal-π organic linker bond should be described with sufficiently accurate quantum chemical methods. Unfortunately electronic structure methods that are routinely used today do not meet the requirements in terms of accuracy. It is known that precisely this metal-organic connection is the weak point of the material, leading to deformations and eventually breakage of the framework.
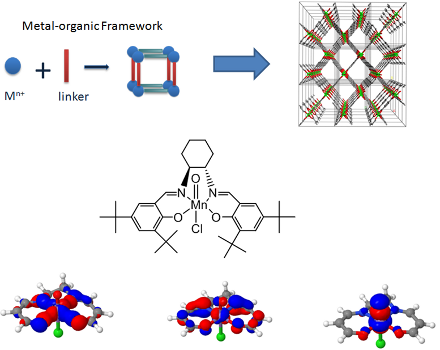
Since the 1930s, the quantum theory of molecular electronic structure is completely known. For certain classes of molecules, typically when π-conjugation or transition metals come into play, Hartree-Fock theory fails completely. A multi-reference description based on several important Slater determinants is required to obtain meaningful results. The energy difference between the exact and the Hartree-Fock solution is called the correlation energy and can be divided into two contributions: static and dynamic correlation. Static correlation can be resolved by calculating the electronic structure exactly in the so-called active space. This is a small subset of orbitals relevant to the chemical description of the molecule under study. One can think, for example, about π-conjugated molecules and the set of π-orbitals relevant to describe its resonant Lewis structures. The orbitals in this active space can be optimized with the complete active space self-consistent field (CASSCF) method. In addition to static correlation, an adequate description of dynamic correlation is required as well to predict energy differences between different deformations at experimental accuracy. Dynamic correlation arises due to the Coulomb repulsion between the electrons in the remaining occupied HF orbitals, which causes small partial occupations in the occupied and virtual orbitals. The most widely used method to calculate dynamic correlation energies for CASSCF wavefunctions is second order perturbation theory (CASPT2), in which a generalized Fock operator is used as zeroth order operator in Rayleigh-Schrödinger perturbation theory.
The electronic structure can be calculated with exact diagonalization in active spaces of up to 16 electrons in 16 orbitals. Quite often, for example to describe metal-π organic linker bonds, the active spaces of interest are much larger. At the Center for Molecular Modeling, a symmetry-adapted high-performance implementation of the density matrix renormalization group (DMRG) was developed, called CheMPS2 [1,2]. This method allows to resolve the electronic structure exactly in complete active spaces of up to 40 electrons in 40 orbitals, and can replace exact diagonalization in the CASSCF (DMRG-SCF) and CASPT2 (DMRG-CASPT2) methods. In 2014 we used DMRG-SCF to provide a better description of the electronic structure of the oxo-Mn(salen) complex, an important industrial catalyst, which is known to be a very challenging problem for electronic structure methods [3]. Figure 1 shows a few orbitals in the active space with significant partial occupation. From left to right are shown: the bonding orbital of the Mn atom with its (N,N,O,O) bridge, the interaction of an Mn d-orbital with the organic π-conjugated backbone, and a bonding orbital of Mn with the axial O atom.
Goal
Within this master thesis, you will learn about conventional (CASSCF and CASPT2) and novel (DMRG, DMRG-SCF, DMRG-CASPT2) electronic structure methods for multi-reference problems and how to use our in-house DMRG code CheMPS2 (which contains the DMRG-SCF and DMRG-CASPT2 methods). You will perform a thorough investigation of the electronic structure and the strengths of metal-π organic linker bonds in MOFs.
Context
The application of approximate yet accurate electronic structure methods in a high-performance computing environment, lies at the border between engineering and physics, as skills and concepts from both fields are needed to successfully accomplish the thesis goals.This research topic will be conducted in the framework of a strong international network and if possible the student will be actively involved in work discussions with collaborative partners. This research topic will be conducted in the framework of a strong international network and if possible the student will be actively involved in work discussions with collaborative partners.
- Study programmeMaster of Science in Engineering Physics [EMPHYS], Master of Science in Physics and Astronomy [CMFYST]ClustersFor Engineering Physics students, this thesis is closely related to the cluster(s) FUNDAMENTALS, MODELINGKeywordsMolecular electronic structure methods for multi-reference problems, Metal-organic frameworksReferences
[1] S. Wouters, W. Poelmans, P. W. Ayers and D. Van Neck. CheMPS2: a free open-source spin- adapted implementation of the density matrix renormalization group for ab initio quantum chemistry. Computer Physics Communications 185 (6), 1501-1514 (2014), http://dx.doi.org/10.1016/j.cpc.2014.01.019 or http://arxiv.org/abs/1312.2415
[2] S. Wouters, CheMPS2: a spin-adapted implementation of DMRG for ab initio quantum chemistry (2013-2016), https://github.com/sebwouters/chemps2
[3] S. Wouters, T. Bogaerts, P. Van Der Voort, V. Van Speybroeck and D. Van Neck, Communication: DMRG-SCF study of the singlet, triplet, and quintet states of oxo-Mn(Salen), Journal of Chemical Physics 140, 241103 (2014), http://dx.doi.org/10.1063/1.4885815 or http://arxiv.org/abs/1405.5642

