Molecular engineering of active sites to increase propene selectivity and catalyst lifetime under MTH conditions
Molecular engineering of active sites to increase propene selectivity and catalyst lifetime under MTH conditions
Promotor(en): V. Van Speybroeck /16NANO01 / Nanoporous materialsEthene and propene are elementary building blocks for the production of base chemicals and polymers. Such light olefins are traditionally produced from thermal naphtha cracking and catalytic cracking of heavy crude oil fractions. Alternatively, these products can also be synthesized from alcohols – originating from sustainable resources – over acidic zeolitic materials. The conversion of methanol to hydrocarbons (MTH) received a lot of attention during the last decades due to its relevance in the search for alternative processes to produce hydrocarbon products. The complex MTH reaction occurs in several stages (Figure 1). A crucial point is that a hydrocarbon pool (HP) is formed, taking the role as co-catalyst for product formation. Indeed, it is generally believed that the MTH catalyst has a supramolecular nature, meaning that next to the zeolite’s Brønsted acid site, also organic molecules occluded in the pores catalyze the reactions. This HP consists of aromatic species such as poly-methylated benzenes (PMB) and the alkenes themselves (Figure 1).
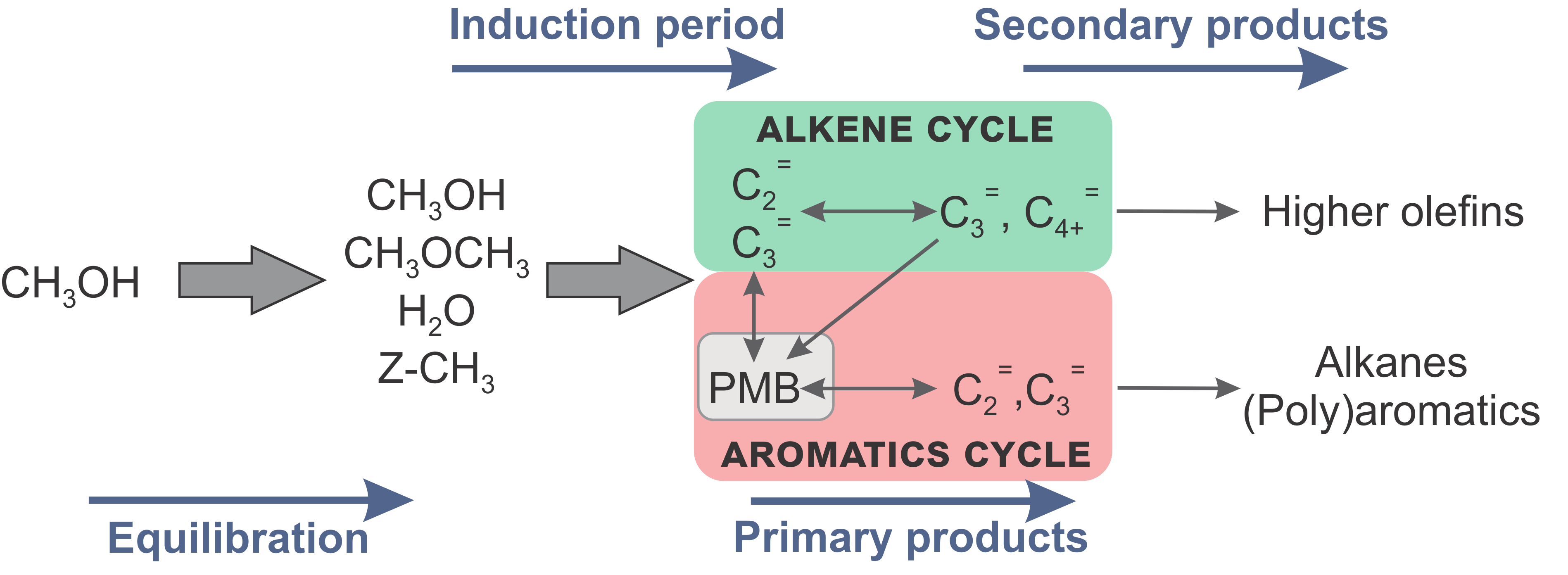
Figure 1. Schematic representation of the reaction stages during MTH conversion. The colored areas highlight the dual cycle concept.PMBs and alkenes play active roles in distinct catalytic cycles (Figure 1), each with a distinct product spectrum. While the aromatic cycle mainly results in ethene, alkanes and (poly)aromatics, propene and larger linear and branched alkenes are typical products of the alkene cycle. Depending on the catalyst topology and reaction conditions both cycles can exist next to each other, which is called the dual cycle concept. Despite the many efforts that have been undertaken to elucidate the MTH mechanism, it is striking that many details concerning the catalytic cycles responsible for olefin elimination have not been revealed yet. There are however indications that the dual cycle concept is one of the underlying engines that determines product selectivity of methanol conversion. It has been shown that selectivity tuning of the MTH process can be achieved by the promotion or inhibition of one of the two catalytic cycles. In view of increasing propene demands, industry is more and more focused on flexible and efficient propene-on-demand processes. One strategy to achieve this is by modifying the zeolite’s active sites through incorporation of phosphorus, boron, transition metals or alkaline earth metals. The interest in this topic revives due to very promising recent experimental results on Ca-ZSM-5 of one of the CMM’s partner laboratories.
Goal
The first goal of this master thesis is to identify potential candidates in the series of earth alkaline metals for zeolite modification. Such modifications might cause chemical and textural modifications of the catalyst and were found to result in higher propene selectivity and longer catalyst lifetime during methanol conversion. Despite the many experimental studies in this field, molecular level insights are lacking. In this view, a rigorous study of alkaline earth modified zeolites will be performed to provide solid proof of the nature of the active site in alkaline earth metal modified ZSM-5. In particular, by means of a combination of high level static DFT calculations and MD simulations the stability and activity of several Brønsted and Lewis acid sites will be assessed. This study will be guided by catalytic and spectroscopic data provided by experimental partners. Finally, it will be assessed to what extent such modifications impose spatial constraints in the zeolitic pores. Depending on the precise form under which the alkaline earth metals are present in the zeolitic pores, it can be expected that such species can block pores and impose spatial constraints. Therefore a systematic comparison of modifications with alkaline earth metals with increasing ionic radii in the series Mg2+
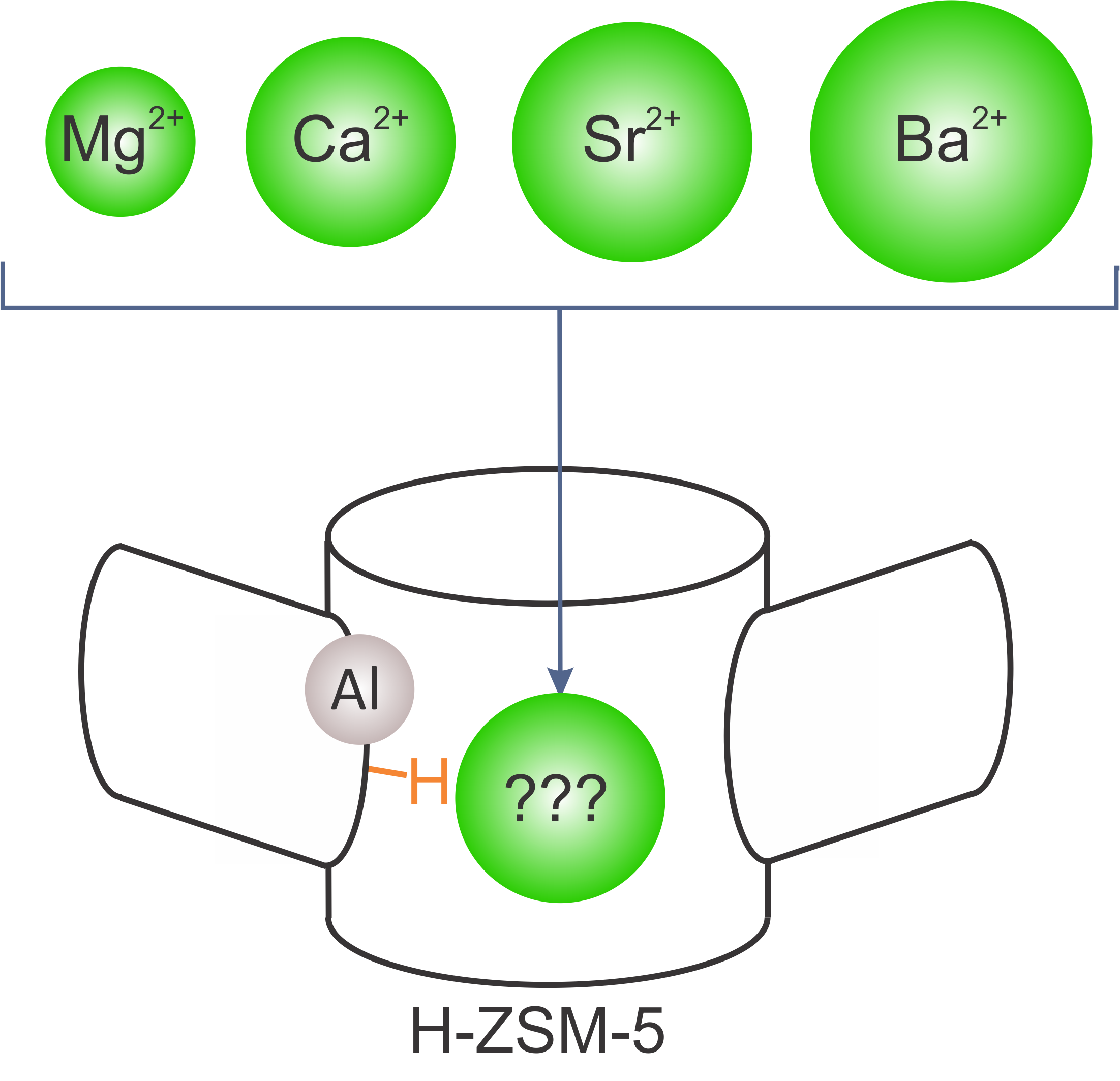
Figure 2 Possible alkaline earth metal modifications of H-ZSM-5.The Center for Molecular Modeling has experience with the studied system and has built up vast expertise in advanced simulation techniques. It is the intention to involve the student actively in the work discussions with our experimental collaborators from the group of Prof J. Gascon’s (Delft University of Technology) who will provide experimental data on this topic. The CMM has access to sufficient computational resources to execute this research project. The proposed topic is challenging and requires technical skills, creativity and chemical insight.
- Study programmeMaster of Science in Chemical Engineering [EMCHEM]KeywordsZeolites, Heterogeneous Catalysis, Chemical kinetics, Computational applicationsRecommended coursesMoleculaire modellering van industriële processen, Simulations and Modeling for the Nanoscale


