Abstract
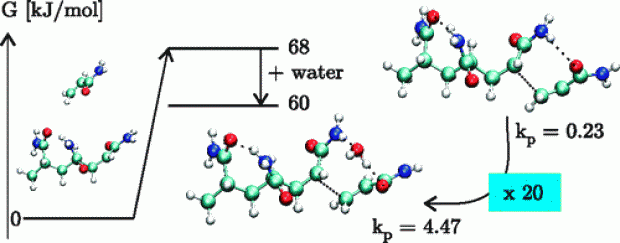
The polymerization of acrylamide (AA) and methacrylamide (MAA) was studied by an extensive set of computational methods with a particular focus on the possible influence of water molecules on the propagation reaction. An extensive set of electronic structure methods was tested, consisting of B3LYP, BMK, MPWB1K, MP2, and B2-PLYP of which some include dispersion effects. The effect of water on the transition state is modeled in two different ways. Explicit water molecules are added to the system, showing that replacing the hydrogen bond that dominates the transition state structure by a water-mediated hydrogen bond, results in more stable, more feasible transition states. This effect is the largest for AA polymerization, a monomer that is known to experience a larger solvent effect than MAA. Additionally, a conductor-like polarizable continuum model (C-PCM) is applied on both the transition states in gas phase and the ones bearing explicit water molecules. This model has a dramatic effect on all the propagation rates, raising them by about 3 orders of magnitude. The inclusion of explicit water molecules gives insight into the role of water molecules and the formation of prereactive complexes. The relative rate of polymerization of AA with regard to MAA is well reproduced for a trimeric propagating radical with inclusion of explicit water molecules or by using an implicit solvation model at the BMK and MPWB1K level of theory.
