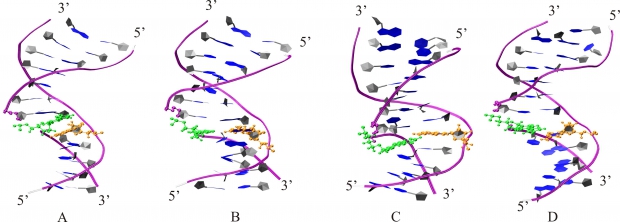

Abstract
Oligodeoxynucleotides incorporating a reactive functionality can cause irreversible cross-linking to the target sequence and have been widely studied for their potential in inhibition of gene expression or development of diagnostic probes for gene analysis. Reactive oligonucleotides further show potential in a supramolecular context for the construction of nanometer-sized DNA-based objects. Inspired by the cytochrome P450 catalyzed transformation of furan into a reactive enal species, we recently introduced a furan-oxidation-based methodology for cross-linking of nucleic acids. Previous experiments using a simple acyclic building block equipped with a furan moiety for incorporation into oligodeoxynucleotides have shown that cross-linking occurs in a very fast and efficient way and that substantial amounts of stable, site-selectively cross-linked species can be isolated. Given the destabilization of duplexes observed upon introduction of the initially designed furan-modified building block into DNA duplexes, we explore here the potential benefits of two new building blocks featuring an extended aromatic system and a restored cyclic backbone. Thorough experimental analysis of cross-linking reactions in a series of contexts, combined with theoretical calculations, permit structural characterization of the formed species and allow assessment of the origin of the enhanced cross-link selectivity. Our experiments clearly show that the modular nature of the furan-modified building blocks used in the current cross-linking strategy allow for fine tuning of both yield and selectivity of the interstrand cross-linking reaction.
