Quasiparticle properties in a density-functional framework
Abstract
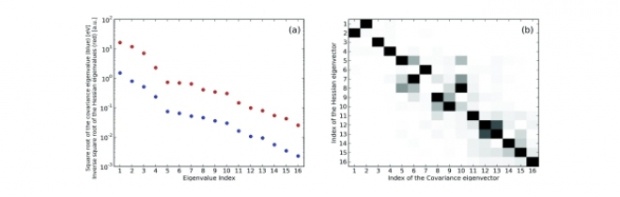
We propose a framework to construct the ground-state energy and density matrix of an N-electron system by solving a self-consistent set of single-particle equations. The method can be viewed as a nontrivial extension of the Kohn-Sham scheme (which is embedded as a special case). It is based on separating the Green’s function into a quasiparticle part and a background part, and expressing only the background part as a functional of the density matrix. The calculated single-particle energies and wave functions have a clear physical interpretation as quasiparticle energies and orbitals.

 Open Access version available at
Open Access version available at