S. Catak, M. D'Hooghe, T. Verstraelen, K. Hemelsoet, A. Van Nieuwenhove, H-J. Ha, M. Waroquier, N. De Kimpe, V. Van Speybroeck
Journal of Organic Chemistry
Abstract
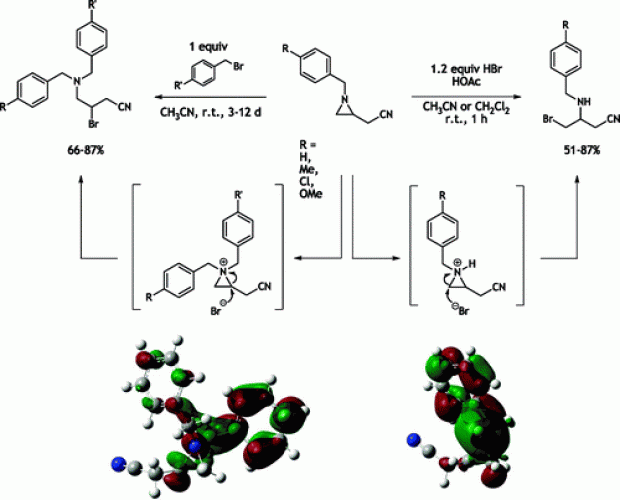
Ring opening of 1-arylmethyl-2-(cyanomethyl)aziridines with HBr afforded 3-(arylmethyl)amino-4-bromobutyronitriles via regiospecific ring opening at the unsubstituted aziridine carbon. Previous experimental and theoretical reports show treatment of the same compounds with benzyl bromide to furnish 4-amino-3-bromobutanenitriles through ring opening at the substituted aziridine carbon. To gain insights into the regioselective preference with HBr, reaction paths have been analyzed with computational methods. The effect of solvation was taken into account by the use of explicit solvent molecules. Geometries were determined at the B3LYP/6-31++G(d,p) level of theory, and a Grimme-type correction term was included for long-range dispersion interactions; relative energies were refined with the meta-hybrid MPW1B95 functional. Activation barriers confirm preference for ring opening at the unsubstituted ring carbon for HBr. HBr versus benzyl bromide ring opening was analyzed through comparison of the electronic structure of corresponding aziridinium intermediates. Although the electrostatic picture fails to explain the opposite regiospecific nature of the reaction, frontier molecular orbital analysis of LUMOs and nucleophilic Fukui functions show a clear preference of attack for the substituted aziridine carbon in the benzyl bromide case and for the unsubstituted aziridine carbon in the HBr case, successfully rationalizing the experimentally observed regioselectivity.

 Open Access version available at
Open Access version available at