Synthesis, Structural Characterization, and Catalytic Performance of a Vanadium-Based Metal-Organic Framework (COMOC-3)
Abstract
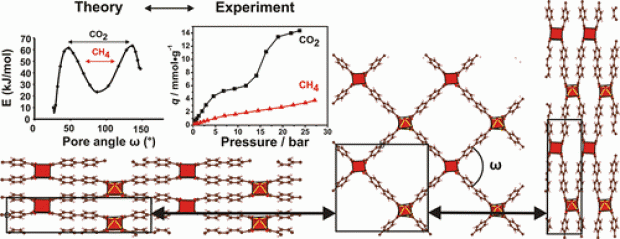
A vanadium 2,6-naphthalenedicarboxylate, VIII(OH)(O2C–C10H6–CO2)·H2O, denoted as COMOC-3as (COMOC = Center for Ordered Materials, Organometallics and Catalysis, Ghent University), has been synthesized under hydrothermal conditions by means of both a solvothermal and a microwave synthesis procedure. The structure shows the topology of an aluminium 2,6-naphthalenedicarboxylate, the so-called MIL-69 (MIL = Materials of the Institute Lavoisier). After calcination at 250 °C in air, the VIII center was oxidized to VIV with the structure of VIVO(O2C–C10H6–CO2) (COMOC-3). The oxidation process was verified by cyclic voltammetry and EPR spectroscopy. The crystallinity was investigated by variable-temperature XRD. The title compound is stable against air and moisture. The catalytic performance of COMOC-3 was examined in the liquid-phase oxidation of cyclohexene. COMOC-3 exhibited similar catalytic performance to MIL-47 [VO(O2C–C6H4–CO2)]. The compound is reusable and maintains its catalytic activity through several runs.